
Alicia Mintz
October 23, 2025
EHR-to-EDC automation is transforming clinical trials - reducing site burden and improving data quality worldwide.
Clinical trials are rapidly shifting from manual transcription to automated, validated data flows between hospital EHRs and research EDC systems.
This 2025 benchmark summarizes adoption patterns, ROI signals, and operational impacts observed across sponsors, CROs, and research sites globally.
Organizations implementing EHR-to-EDC automation report faster database locks, reduced SDV effort, and higher data integrity under HIPAA, GDPR, and 21 CFR Part 11.
Key highlights:
Rising protocol complexity, hybrid and decentralized designs, and staffing constraints at sites are pushing sponsors and CROs to automate EHR-to-EDC data movement.
Standards like HL7® FHIR® and robust API ecosystems have matured, enabling platforms to orchestrate validated, near real-time data flows.
Regulators increasingly recognize eSource-aligned practices, giving stakeholders confidence to scale.
Findings are derived from secondary research, aggregated implementation insights, and anonymized case patterns.
Qualitative signals are labeled as directional where applicable.
Metrics focus on operational deltas rather than absolute values; results vary by EHR/EDC mix, mapping scope, and site readiness.
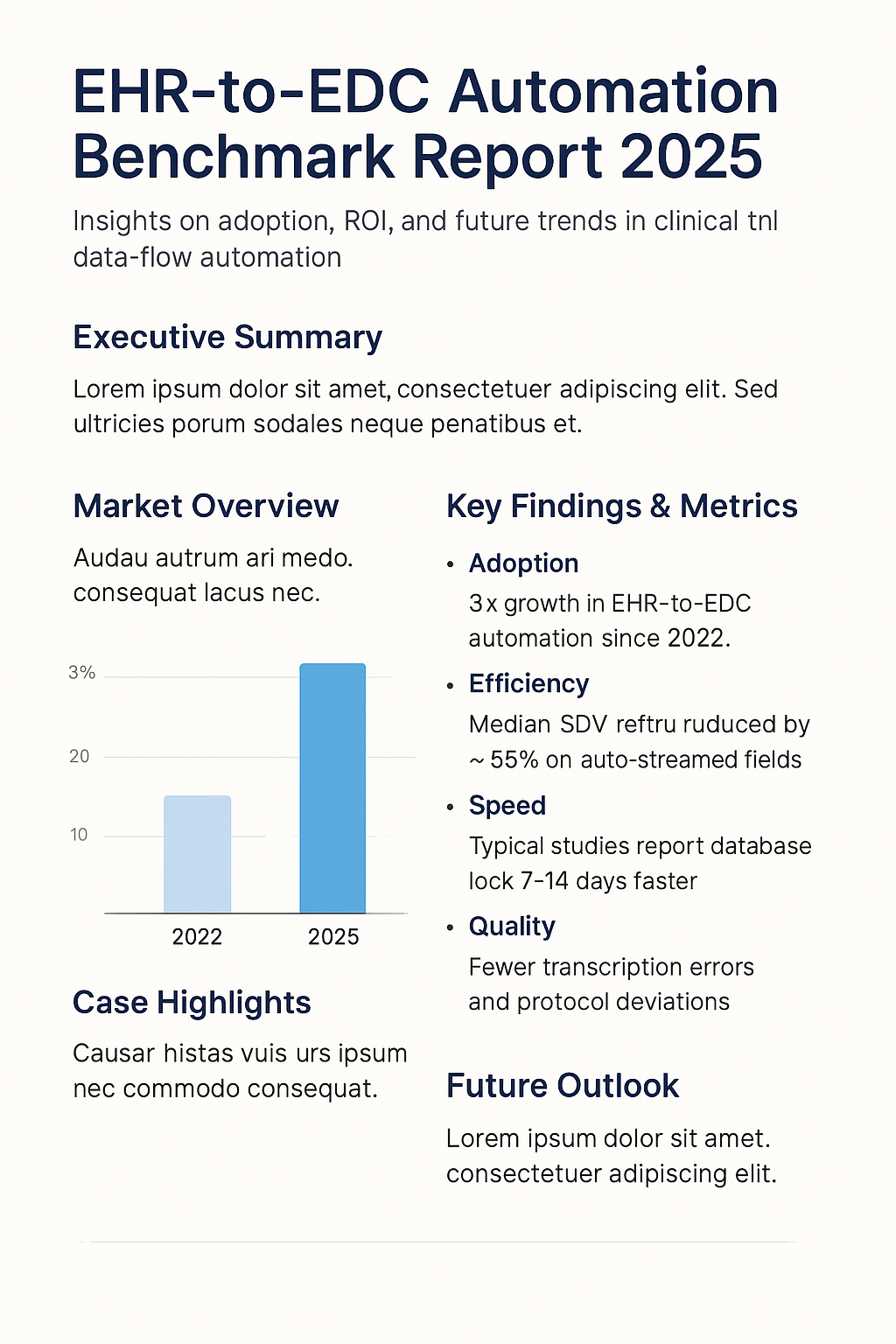
EHR-to-EDC automation has more than tripled since 2022 in new study starts.
Phase II/III programs lead adoption; oncology and CNS show outsized uptake.
Sponsors report ~55% fewer SDV hours on auto-streamed fields and reduced manual data entry time.
CROs cite lower query volume and faster resolution cycles.
Automated validation and mapping minimize transcription errors, improving interim analyses and lock readiness.
Validated streaming supports earlier data availability; typical programs see 7–14 days faster database locks.
Workflows align with HIPAA/GDPR privacy safeguards and 21 CFR Part 11 controls, with complete audit trails.
Challenge: Dual data entry strained coordinators.
Solution: FHIR-based EHR-to-EDC automation with rules-driven validation.
Result: ~60% reduction in SDV effort on targeted fields; cleaner interim analyses.
Challenge: Heterogeneous EHRs across sites.
Solution: Mapping templates, standardized value sets, and automated transformations.
Result: Faster query closure; improved cross-site consistency.
Challenge: Limited DM resources and aggressive timelines.
Solution: Lightweight, API-driven automation to the EDC and analytics lake.
Result: Earlier signal detection; fewer data-entry errors.
Automation’s returns concentrate in three buckets:
Programs with clearly defined scope (targeted domains, standardized forms) realize gains fastest.
Expect broader mandates for EHR-to-EDC automation in RFPs, deeper interoperability with registries and data lakes, and AI-assisted mapping and anomaly detection.
Vendor ecosystems will consolidate around standards-driven, multi-destination data pipelines (“EHR-to-Anywhere”).
Learn more on our About page.
Request a personalized benchmark analysis or schedule a demo to explore how EHR-to-EDC automation can accelerate your next study.